Abstract
Background: Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is a disease primarily of older patients (pts) who often have comorbidities and are less able to tolerate aggressive regimens. Quality of life (QOL) is an important consideration; however, QOL data from randomized trials in CLL are limited, particularly in older pts.Ibr is a first-in-class, once-daily inhibitor of Bruton's tyrosine kinase approved for the treatment of CLL and allows for treatment without chemotherapy. In the phase 3 RESONATE-2 study of older pts with TN CLL/SLL, single-agent ibr reduced the risk of progression or death by 84% vs clb (P <0.001), with median 18.4 mo follow up (FU) at primary analysis (PCYC-1115; Burger N Engl J Med 2015). We report updated data with a focus on QOL and measures of well-being with extended FU in RESONATE-2.
Methods: Pts ≥65 years were randomized 1:1 to receive 420 mg ibr once daily until progressive disease (PD) or clb for up to 12 months. At PCYC-1115 closure, consenting pts were transferred to PCYC-1116. Pts with PD on clb could receive second-line ibr. In PCYC-1115/1116 (collectively RESONATE-2), PROs of FACIT-Fatigue (F) and EQ-5D-5L were measured by change from baseline to each assessment time. Quality-adjusted time without symptoms or toxicity (Q-TWiST) was determined by partitioning of overall survival using Kaplan-Meier methods, and was also weighted by PRO using EORTC QLQ-C30 global health status (for PCYC-1115 only).
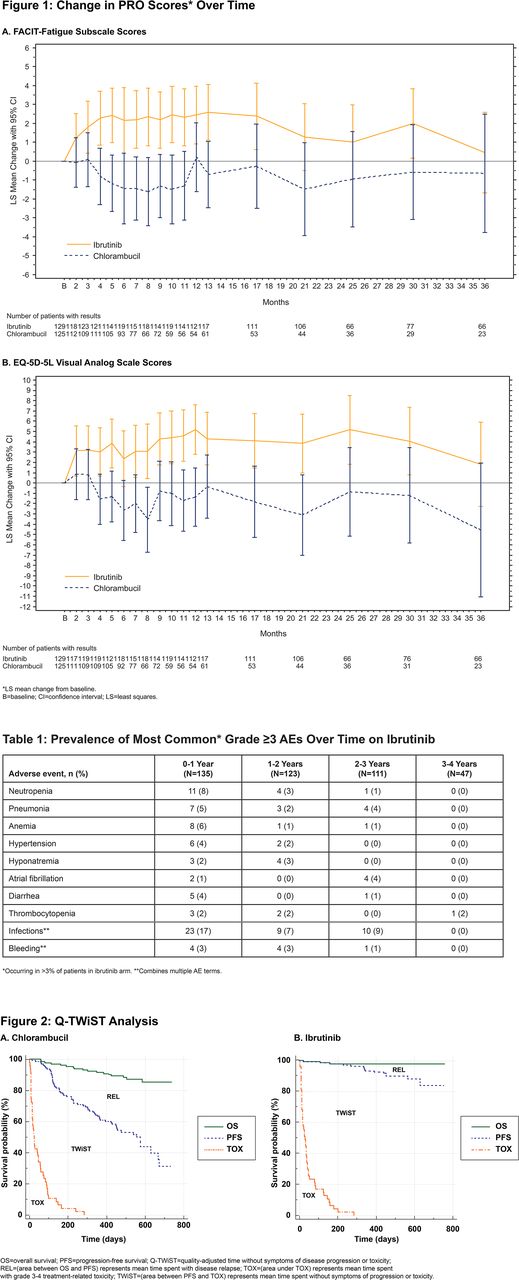
Results: Among 269 pts, reasons for initiating therapy included progressive marrow failure (38%), lymphadenopathy (37%), splenomegaly (30%), fatigue (27%), and night sweats (25%). Median age was 73 years; 69% had comorbidities (CIRS score >6, reduced creatinine clearance, or ECOG status 2). Median FU was 35.7 mo with ibr and 34.4 mo with clb. Ibr resulted in significantly longer PFS (median, not reached vs 15.0 mo with clb), with an 87% reduction in risk of progression or death vs clb (HR 0.130; 95% CI: 0.081, 0.208). PFS rate at 30 mo was 85% with ibr vs 28% with clb. Greater and sustained improvements in PROs were observed with ibr vs clb with significantly greater improvements over time vs clb in FACIT-F (P=0.0021; Figure 1A) and EQ-5D-5L Visual Analogue Scale (P=0.0004; Figure 1B) by repeated measures. In clb pts with PD, PROs improved after crossing over to ibr. Approximately 87% of pts on ibr (vs 52% on clb) had decreased/normalized lymphadenopathy within 2 mo, which was sustained through 36 mo. Disease symptoms, including fatigue and night sweats, improved more frequently vs clb. A greater proportion of pts with baseline cytopenia showed sustained hematologic improvement with ibr vs clb for hemoglobin (90% vs 45%; P <0.0001) and platelets (83% vs 46%; P=0.0032). Median treatment duration was 34.1 mo on ibr vs 7.1 mo on clb. Medical resource utilization burden was less with ibr vs clb in the first year (use of intravenous immunoglobulin, growth factors, or transfusions), and subsequently continued to decrease. The most common adverse events (AEs) of any grade with ibr were diarrhea (47%), fatigue (33%), and cough (30%). Eight grade ≥3 AEs had a prevalence of >3% in ibr pts and generally decreased or were stable over time (Table 1). During the first year of treatment, pts on ibr vs clb experienced less grade ≥3 neutropenia (8% and 18%) and anemia (6% and 8%); other common grade ≥3 AEs were pneumonia (5% and 2%) and hypertension (4% and 0%). Grade ≥3 bleeding occurred in 7% of ibr pts over the 3 yr FU. AEs leading to treatment discontinuation occurred in 16% with ibr over 34 mo vs 23% for clb over 7 mo of therapy, respectively. Based on Q-TWiST analysis at primary analysis (median FU 18.4 mo), mean time spent without symptoms of PD or grade 3-4 treatment toxicity was longer with ibr vs clb (501 vs 351 days; mean difference, 150 days; 95% CI: 109, 193) (Figure 2). Pts on ibr therapy also experienced prolonged PRO quality-adjusted survival compared with clb (386 vs 329 days; mean difference, 57 days; 95% CI: 25, 90).
Conclusions: With 3 yrs of FU in pts with TN CLL/SLL, ibr compared with clb continued to show greater and sustained improvements in PRO, and more frequent improvements in other measures of well-being including disease burden and hematologic parameters, which were common IWCLL indications for therapy. Ibr was also associated with increased quality-adjusted survival time vs clb at time of primary analysis.
Tedeschi: AbbVie: Consultancy; Gilead: Consultancy, Other: travel expenses; Janssen: Consultancy, Other: travel expenses. Owen: AstraZeneca: Honoraria; Celgene: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; Gilead: Honoraria, Research Funding; Lundbeck: Honoraria; Roche: Consultancy, Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding. Robak: Pharmacyclics LLC, an AbbVie Company: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Akari Therapeutics Plc: Research Funding. Barr: Celgene: Consultancy; Gilead: Consultancy; Seattle Genetics: Consultancy; Novartis: Consultancy; Infinity: Consultancy; AbbVie: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding. Bairey: Janssen: Consultancy, Research Funding. Hillmen: Roche: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Celgene: Research Funding; GSK: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding. Coutre: AbbVie: Consultancy, Research Funding; Janssen: Consultancy; Celgene: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding. Devereux: GSK: Consultancy; Roche: Consultancy, Other: travel expenses; MSD: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Servier: Other: Advisory board; Gilead: Consultancy, Honoraria, Other: travel expenses, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: travel expenses, Speakers Bureau. McCarthy: AbbVie: Honoraria, Other: travel expenses; Chugai: Other: travel expenses; Celgene: Other: travel expenses; Roche: Consultancy, Other: travel expenses; Janssen: Consultancy, Honoraria, Other: travel expenses; Novartis: Honoraria, Other: travel expenses. Simpson: Celgene: Honoraria, Other: travel expenses; Amgen: Research Funding; Roche: Honoraria; Pharmacyclics LLC, an AbbVie Company: Research Funding; Onyx: Research Funding. Siddiqi: Juno: Other: Steering committee for JCAR017; Seattle Genetics: Speakers Bureau; Pharmacyclics, an AbbVie Company: Other: Steering committee for ibrutinib, Speakers Bureau. Iyer: Optimal Strategix Group: Patents & Royalties: Self at Optimal Strategix Group; Eli Lilly: Other: Brother at Lilly. Lal: AbbVie: Equity Ownership; Gilead Sciences: Employment; The Permanente Medical Group (TPMG): Employment; Pharmacyclics LLC, an AbbVie Company: Employment. Trudeau: Janssen Global Services, LLC: Employment, Equity Ownership. Dai: Pharmacyclics, an AbbVie Company: Employment; AbbVie: Equity Ownership. Dean: CTI BioPharma Corp: Employment, Equity Ownership, Other: Employment of immediate family member; AbbVie: Equity Ownership; Pharmacyclics LLC: Employment, Other: Employment of immediate family member. James: AbbVie: Equity Ownership; Pharmacyclics LLC, an AbbVie Company: Employment. Burger: Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Novartis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses. Ghia: Adaptive: Consultancy; Gilead: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy; Roche: Consultancy; AbbVie: Consultancy; Novartis: Research Funding. Kipps: Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Research Funding; Oncternal: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal